In addition to powder coating, electroplating is a popular surface treatment process for metal finishing that is often used in a variety of industries. So what is electroplating and how does it work and what industries can it be applied to, this article will tell you the answers.


What Is Electroplating?
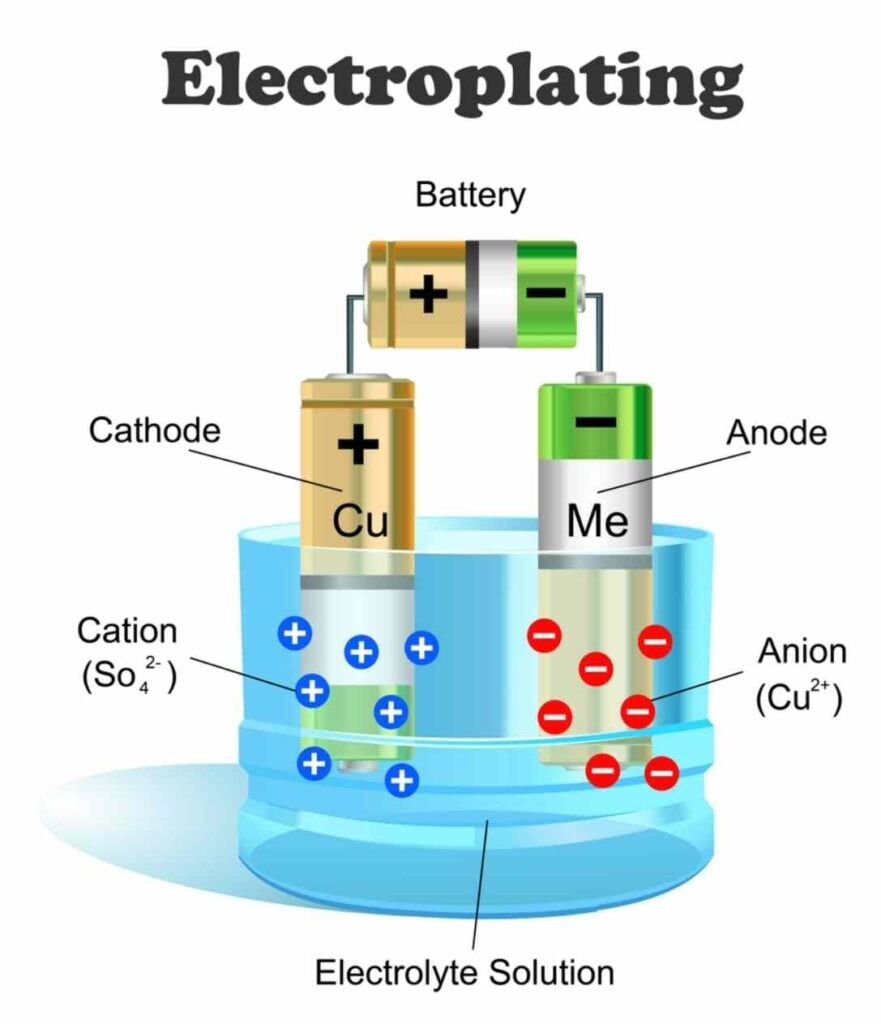
Electroplating is the process of applying a metal coating to an element for various purposes. Typically, electroplating is done to alter an object’s aesthetic appeal, increase durability, enhance corrosion resistance, and improve electrical conductivity. Electroplating is a form of electrolysis where the metal ions are deposited onto the substrate in the form of a thin and uniform layer.
Largely, industries such as automotive and electronics utilize electroplating within their processes to enhance the functionality and the service life of their products. For example, jewelry that is made with a gold coating is not susceptible to tarnishing, while chromium automotive components endure extreme environment.
What is the purpose of electroplating?
- Proection: Prevents corrosion, physical damage, and exposure to chemicals.
- Aesthetics: Provides a decorative finishing to pieces of jewelry, parts of cars, and even household items.
- Functionality: Increases conductivity of electronics or lessens friction within mechanical parts.
What Is the Electroplating Process?
The electroplating process breaks down into four steps:
Step one – Surface Preparation • The substrate (base material) goes through a cleaning process which removes oxides, grease, or dirt. Options include abrasive blasting, acid dipping, or ultrasonic cleaning.
Step two – Electrolyte Solution Preparation • The preparation of electrolyte involves preparing a solution that contains all the dissolved ions of the coating metal. For instance, the sulfamate nickel solution is used as the electrolyte in nickel plating.
Step three – Electrolysis • The substrate (cathode) and the coating metal (anode) are dipped in the electrolyte. • The substrate and the coating metal are dipped into the electrolyte solution. • A direct current (DC) is passed, with the substrate at negative potential and the coating metal at positive potential. The ions of the metal begin migrating to the cathode and bonding with it.
Step four – Post Treatment Process • The final object undergoes rinsing, polishing, or heat treatment to improve the adhesion and surface finish.

Most Utilized Metals for Electroplating
Different metals serve different needs because of the distinct properties they possess.
• Nickel: Used in automotive trim and kitchenware. Provides smooth finishing along with resistance to corrosion.
• Gold: Used in jewelry and electronics (connectors, circuit boards) because of its exemplary conductivity and luster.
• Silver: Used in mirrors and cutlery because of its beautiful reflectance, as well as for low resistance electrical contacts.
• Chromium: Found in tools, faucets, and car parts due to its shiny appearance and added hardness.
• Zinc: Economically used in galvanization of steel in order to make it rust resistant, though it is often not the most effective.
• Copper: Used as an undercoat in multilayer plating for better adhesion.
What Factors Affect Electroplating?
Several variables influence the quality and efficiency of electroplating:
Current Density
Higher current speeds up plating but risks uneven deposits. Organizations like the National Association for Surface Finishing (NASF) emphasize optimizing current for consistency.For instance, complex parts may require pulsed currents to ensure uniform coating in recessed areas.
Temperature
The temperature of the electrolyte solution affects ion mobility and chemical reactions. Warmer solutions (typically 40–60°C for nickel plating) enhance ion movement, leading to smoother, denser coatings. However, excessive heat can degrade organic additives in the electrolyte, causing defects like pitting or discoloration. Temperature control is critical in processes like chromium plating, where deviations as small as 2°C can alter coating hardness, as noted in ASTM International’s B650 standard for decorative chrome finishes.
Electrolyte Composition
The electrolyte’s chemical makeup determines plating efficiency and coating properties. Impurities (e.g., dissolved metals or organic contaminants) disrupt ion balance, leading to dull or porous finishes. For example, copper electroplating baths require precise sulfate-to-copper ratios to maintain conductivity.
Surface Preparation
A pristine substrate surface is non-negotiable for strong adhesion. Oils, oxides, or microscopic debris create barriers between the substrate and coating, resulting in peeling or blistering. Pre-treatment steps like alkaline degreasing, acid pickling, and ultrasonic cleaning are standard.
Applications of Electroplating
Automotive Industry
The electroplating procedure also aids the cosmetic appeal of engines with chrome trimmings for aesthetic appeal, along with adding zinc plating that helps in the prevention of rust in brakes. Coated nickel on an engine part aids in wear reduction enabling an increase in the vehicle’s life and performance in harsh environments.
Electronics
For smartphones and circuit boards, the reliable transmission of signals is achieved with gold connectors. The conductivity of semiconductors that are important for 5G technology and speedy computation is improved with silver electroplating.
Medical Devices
Surgical instruments that are silver coated are sterile and are anti-bacterial. The use of titanium coatings on bone and heart implants improves the degree of biocompatibility and lowers the risk of rejection. Radiopaque coverings on X-ray machines are done by electroplating too.
Jewelry and Luxury Goods
To obtain a shine that is scratch-resistant, rhodium is used in a coating on white gold, thereby elevating a white gold ring’s beauty. Designer’s watches and eyeglasses have an added coating of gold or platinum, giving timless class while enhancing the durability.
Aerospace
The turbine blades are coated with cobalt-nickel alloys to protect them from high temperature corrosion. Cadmium is used in aircraft fasteners to prevent corrosion due to the exposure of salt water.
Home Decor & Hardware
Faucets and door handles made of chrome do not tarnish. Copper adds a vintage appeal to the lighting fixtures and along with that, prevents oxidation.
Energy Sector
To prevent bolts of wind turbines from rusting in costal areas, zinc-nickel coatings are used. Electroplated solar panel contacts greatly enhance efficiency by adding elements that decrease the electrical resistance of contacts.
Defense & Military
The hard chromium plating on firearm barrels increases durability against wear and tear. Electroless nickel coatings on submarine parts protect against corrosion from seawater.
Optical Industry
Anti reflective silver or aluminum coatings on camera lenses and telescope eyepieces enhance the transmission of light. Electro casting also strengthens the precision laser components.


Benefits of Electroplating
• Corrosion Resistance: Enhances durability in difficult conditions.
• Aesthetic Appeal: Provides shiny and decorative surfaces.
• Improved Conductivity: Important for electrical and electronic parts.
• Wear Resistance: Abrasive resistant coatings safeguard equipment.
• Cost Efficiency: Metal coatings are cheaper than solid metallic components.
• Sustainability: Waste and resource consumption is reduced through recyclable coatings.
Elevate Your Products with Expert Electroplating
Professional plating can make your products more resistant to corrosion and better protected.Ready to enhance your products?Contact our electroplating specialists today for a customized solution that meets your industry’s toughest standards.
Frequently Asked Questions (FAQ)
Q:How long does an electroplated coating last?
A:Lifespan depends on the metal, thickness, and environment. For example, zinc coatings on bolts last 5–20 years, while hard chrome on industrial machinery can endure decades with proper maintenance.
Q: What’s the difference between electroplating and electroless plating?
A: Electroplating requires external electric current, while electroless plating uses chemical reactions for deposition. Electroless methods (e.g., nickel-phosphorus) offer uniform coatings on complex shapes but are slower and costlier.
Q:Can electroplating improve electrical conductivity?
A:Absolutely! Gold or silver plating on connectors reduces resistance, critical for high-speed electronics and renewable energy systems like solar panels.
Q:What are the environmental impacts of electroplating?
A:Modern plating plants comply with regulations for wastewater treatment and metal recovery, and organizations such as NASF promote environmentally friendly practices such as trivalent chromium instead of hexavalent chromium.
Q:Can electroplating be used on non-metal surfaces?
A: Yes! Plastics, ceramics, and composites can be electroplated after pre-treatment (e.g., etching or conductive coatings). This is common in automotive trim and electronics housings.